Pharmacist Resources
Your resource for all things CBD Oil
This page is provided for informational purposes only. Nothing on this page is intended to be medical advice. This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Cannabis use has been reported in a myriad of conditions like anxiety, post-traumatic stress disorder, insomnia, nausea, Tourette's syndrome, and epilepsy [1]. However, cannabis also has reported undesired psychoactive and anxiety-inducing side effects. The two main components of cannabis are tetrahydrocannabinol (THC), a hallucinogen, and cannabidiol (CBD), a cannabinoid. CBD oil is a result of trying to obtain the benefits of cannabis without the unwanted side effects, and abuse potential.
CBD oil and CBD products are made of almost pure cannabidiol, a non-psychotic constituent of cannabis and hemp plants, with only trace amounts of THC. Hemp is a legal agricultural product containing no more than 0.3% THC, and is the plant used to make CBD products.

The Endocannabinoid System
The human body produces compounds and receptors similar in conformation to naturally occurring cannabinoids. The endocannabinoid system is made up of two types of cannabinoid receptors (CB1 & CB2) and anandamide, an endogenous ligand of those receptors. CB1 and CB2 are G-coupled protein receptors that exist on plasma membranes and trigger several difference cascades. CB1 receptors are located primarily in the brain, but found other places around the body including smooth muscle. CB2 receptors are found less abundantly than CB1 receptors, and in immune-system associated organs like the spleen [2].
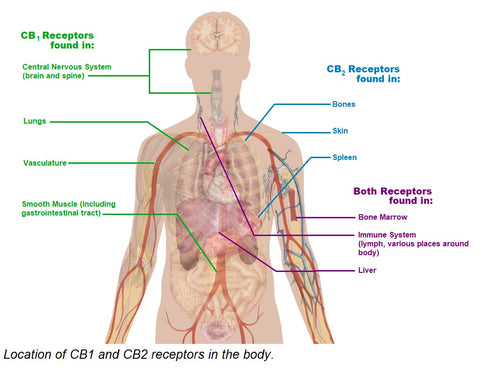
The proposed physiology of CBD Oil
At the CB1 receptor, located mainly in neuronal cells, anandamide and CBD is proposed to affiliate with the G-coupled protein receptor and modulate neurotransmitter release. Excessive neurotransmitter release contributes to anxiety [3]. At the CB2 receptor, located in the periphery of the body, the interaction of anandamide and CBD oil with the receptor is believed to lead to an inhibition of adenylyl cyclase, decreasing cAMP levels, and stimulating beta arrestin to regulate ion channels. This is proposed to interrupt pain signaling and stall the release of pro-inflammatory mediators [3,4].
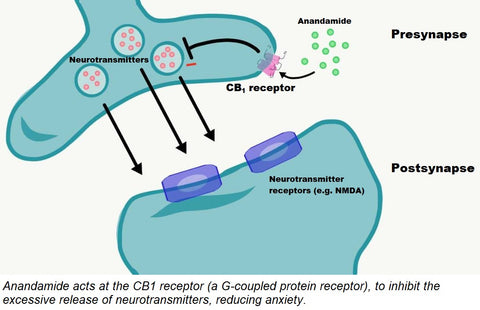
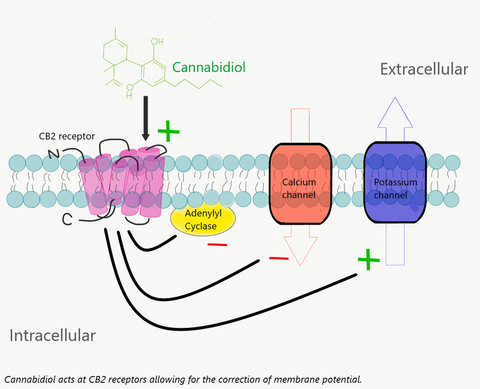
Pharmacokinetics
Food has been demonstrated to enhance the absorption of CBD, yielding an increase in both Cmax and area under the curve of about 4-5 times. The patient does not need to take it with food one way or another, but should be consistent in how they take it. This lipophilic compound has a very large volume of distribution but is also highly protein bound. It is metabolized mainly in the liver, and is a substrate of the Cytochrome P450 system of enzymes, specifically CYP2C19 and CYP3A4. It also has inhibitor and inducer potential for other CYPs. It is has been found to be eliminated fecally, with a half-life of approximately 60 hours [4].
Drug Interactions
Warfarin
Since CBD is metabolized by CYP2C19 and CYP3A4 it competes with warfarin for binding sites, and could potentially increase the amount of warfarin in a patient's system. More frequent INR monitoring upon initiation and dose changes of CBD oil is recommended, as well as the supervision of a healthcare professional [5].
Antiepileptic Drugs
Anti-seizure medications are very tricky because of their mixed effects on the Cytochrome P450 system. CBD oil is metabolized by, and even lightly inhibits, a number of CYPs. While CBD oil may cause some drug levels to rise, some inducers in this category may cause the CBD level to fall. The supervision of a healthcare professional to obtain serum drug levels is recommended [6].
Opioids
A double blind, placebo-controlled study conducted at Mt. Sinai showed no increase in risk of respiratory depression with concomitant CBD Oil use [7]. Due to the likelihood of somnolence while on CBD oil, caution should be used and, as always, it should be used at the discretion of a healthcare professional.
Dose Initiation
When recommending dosing to a patient it is important to take into account titration, their other medications, and what they intend to use cannabidiol for. Dosing will have to be patient specific, therefore starting at lower doses and titrating up to an effective, safe dose is the most effective method we have found. While CBD oil has no FDA approved indications, there is literature on several possible benefits and doses of CBD oil, and for some patients the benefits may occur at doses well below what has been studied. The table below summarized some studies done on cannabidiol.
The main takeaway is that CBD oil has been studied at a range of doses, but patients may need to start low and titrate up the dosage to achieve their desired effect. Doses can be increased weekly, stressing to the patient that the goal is to find the lowest dose that works for them, and we recommend it be taken in the evening until desired clinical effects are realized.
Patients who have a known hypersensitivity to CBD oil or sesame seed oil (vehicle of choice) should not use cannabidiol products. Patients who have known hepatic insufficiency or injury should not use cannabidiol over-the-counter. [8]
For information on ongoing trials, visit clinicaltrials.gov.
References -- Please click the link to visit the reference.
- McPartland, John M., Geoffrey W. Guy, and Vincenzo Di Marzo. “Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions That Upregulate the Endocannabinoid System.” Ed. Andrej A. Romanovsky. PLoS ONE 9.3 (2014): e89566. PMID: 24622769
- Busquets-Garcia, A., Bains J., Marsicano, G. "CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity." Neuropsychopharmacology REVIEWS (2018) 43, 4–20.
- Cannabidiol - Pharmacokinetics. Micromedex Solutions. Truven Health Analytics, Inc. Ann Arbor, MI. Available at: http://www.micromedexsolutions.com.
- Grayson Leslie, Vines Brannon, Nichol Kate, Szaflarski Jerzy P., An Interaction between warfarin and cannabidiol, a case report, Epilepsy & Behavior Case Reports (2017), doi:10.1016/j.ebcr.2017.10.001
- Gedde, Margaret M. "Cannabidiol (CBD) Interactions with Antiepileptic Drugs through Effects on CYP Metabolism." Gedde Whole Health LLC, 2016.
- Manini, Alex F. et al. “Safety and Pharmacokinetics of Oral Cannabidiol When Administered Concomitantly with Intravenous Fentanyl in Humans.” Journal of addiction medicine 9.3 (2015): 204–210.
- Cannabidiol. Micromedex Solutions. Truven Health Analytics, Inc. Ann Arbor, MI. Available at: http://www.micromedexsolutions.com.
- Manini AF, Yiannoulos G, Bergamaschi MM, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. Journal of addiction medicine. 2015;9(3):204-210. doi:10.1097/ADM.0000000000000118.
- Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD)
in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011; 25:121–30. - Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant
schizophrenia. J Psychopharmacol. 2006; 20:683–6. - Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington’s disease.
Pharmacol Biochem Behav. 1991; 40:701–8.
Carolina CBD pharmacy, Myrtle Beach, south carolina
Top